“Cells live in a manifold existence. They are both complex biochemical information processing entities and mechanical objects that live within mechanically active environments. As our knowledge of the molecular mechanisms underlying mechanosensation and mechanotransduction improves, these mechanical and biochemical realities appear increasingly intertwined.”
….
In therapy and training, an essential parameter we manipulate to induce biological tissue adaption with load is “TIME”. In therapy, rehabilitation and training (in reality, they are all load at different scales) our focus is often within a very finite time range at the furthest end of the spectrum (ie. milliseconds to minutes). However, some forces act on the body much longer, even over an entire lifespan (ex. Gravity).
In the context of this article, please be mindful of the term “force dissipation”. I have been guilty in the past of having a conceptual bias toward “force production” or “force transmission”. They may all be true, and even appear synonymous, but I believe “dissipation” is most appropriate as we discuss the passive components of connective tissue as opposed to the contractile components seen in skeletal muscle. Force dissipation is a normal and healthy response to reduce localized tissue overload. At small time-scales (milliseconds to minutes) tissue must dissipate force rapidly to avoid structural failure. However, with exposure over longer time-scales, tissue becomes more proficient at dissipating force through its material properties via biological adaptations, therefore increasing its load-capacity and lowering its failure threshold. This article reviews biological strategies of tissue to dissipate force, from the shortest (milliseconds) to the longest (life time) time scales. Having a better understanding of this time spectrum will help us understand how we as therapists and coaches can utilize force-based inputs with more specificity and precision.


….
Rapid physical perturbations (milliseconds to seconds)


Over these short time-scales, tissue must dissipate force rapidly to avoid structural failure. The
immediate impact of sudden mechanical perturbations on any tissues will be governed by their material properties. To a large extent, this will be dictated by molecular assemblies such as the cytoskeleton and lipid membranes. As a result, alterations in the mechanical properties and organization of the structures can lead to disease (injury). Poro-elastic behavior (a) describes the rapid redistribution of cytosol through networks of organelles and the cytoskeleton to dissipate force within a cell at sub-second time scales.
Another immediate effect is deformation of the plasma membrane and the endomembrane system of the tissue’s cells. Plasma membrane can only withstand 2-3% stretch before lysis – rapid stretching causes high stress and risks rupture. To avoid this, reserves of membrane are stored in folds that flatten upon stretch. Conversely, when the surface area is suddenly reduced, excess plasma membrane forms tubules whose initial shape can be accurately predicted from physical considerations.
Turnover of protein assemblies (second-minutes timescales)
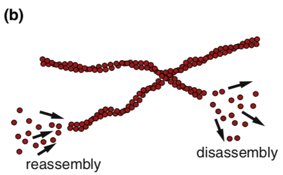

At minute time-scales the turnover of proteinaceous structures that bear the load of externally applied stress will function as a source of stress dissipation (b). Especially in large-scale cytoskeletal networks of filaments that connect cells to their environment, protein turnover will cause the network as a whole to adapt to the new mechanical environment through the remodelling of stress-bearing structures. This process can be actively enhanced by the action of associated motor protein. These features act as a first line of defense against the dangers of acute or prolonged stress by ensuring that internal stresses are rapidly redistributed.
This highlights the utility of active inputs (ie. muscle contractions) in addressing connective tissue pathology to improve force dissipation efficiency. With progression, irradiation techniques can be used to load and train more tissue to dissipate force across a much greater area. This is the time scale in which we begin to see how we can influence the biological constructs of tissue to promote long-term adaptation.
Intermediate time-scales responses stem from cellular process (minutes-hours timescales)
This is the first time-frame over which active cellular behaviours begin to play a role in stress dissipation and in dealing with the consequences of changes in cell structure that arise from an imposed deformation. It is over the course of minutes that the flaws in the crystal lattice of microtubules subjected to cycles of bending can self-heal through the incorporation of new tubulin heterdimers.


There are also a wide variety of cellular behaviours, all of which can function to regulate tissue stress, including: cell neighbour exchange, cell division, cell fusion and cell extrusion. By exchanging neighbours, cells are able to translocate within an epithelium (c) – these rearrangements can alter cell and tissue shape as well as local patterns of stress. This process relies on the ability of cells to add or remove specific cell-cell interfaces via the regulation of junction tension and adhesion.
In Functional Range Release ®, we manipulate force input to encourage cells to respond in a way we desire. When we identify mechanical tension, we apply a directional force so cells may respond to improve force dissipation at the site of load. In mechanical tension, the cellular organization is inefficient at dissipating force. When cells don’t experience directional force, they undergo apoptosis (cell death). Since cells communicate via cell-to-cell junction, neighbouring cells will also apoptos, “shrivel” and aggregate in a disorganized manner. Hence, when we complete a FR soft tissue assessment, we perceive mechanical tension as an area which feels “more tense” due to the tissues reduced ability to dissipate force across that area.


Cell division can also have an effect analogous to neighbour exchange on tissue stress (d). The cell shape changes taking place during mitotic rounding and anaphase elongation enable division to redistribute the mother cell’s mass, causing a dilation along the axis of division and a contraction in the perpendicular direction. This makes the orientation of division critical – divisions are oriented along the long axis of interphase call shape.
Externally applied stress has also been implicated. The application of an anisotropc strain induces alignment of the cell long axes in the principal strain direction, leading to oriented divisions that facilitate the flow of cellular material to dissipate the stress, eventually restoring isotropic cell shape. This may represent a generic cellular mechanism.
Long time-scales (days-years)
At the furthest end of the spectrum, an organism must generate and retain its complex 3D form in the presence of gravity, whose mechanical influence is felt through an entire lifetime. Since cellular tissues behave like viscous fluids over long time scales, a constitutive tension might be expected to drive flows to achieve a reduction in surface area – transforming tissue structures into featureless balls. This can be prevented for example, by balancing constitutive tension with a preferred cell shape, or by attaching tissues to less viscous structures, such as the extracellular matrix or bone. In general, the combination of stable boundaries and patterned tension generation can robustly guide tissue morphogenesis.
In living cells, molecular turnover within force-bearing cellular substructures is almost ubiquitous and will rapidly relax most stresses. Thus, channel opening and protein unfolding can only monitor stresses that arise at shorter time-scales than molecular turnover, limiting their importance in many morphogenetic processes (which act over periods of minutes to days) unless they are functioning as components of a homeostatic system. As explained, over a period of hours to days the processes of cell growth, division, neighbour exchange and extrusion will tend to homeostatically restore cell shape as well as remove tissue stresses.
….
Distinguishing between the complex emergent physical properties of living matter and the equally complex mechanoresponsive behaviours of cells is crucial and presents an exciting challenge for the present and future. As research further discovers this realm, we can begin to abide by the principal cellular laws and apply load to achieve the outcomes we desire, in both training and therapy.
Reference:
Watt T, Baum B and Charra G. A question of time: tissue adaptation to mechanical forces. Current opinions in cell biology. 38:68-73. 2016.

